Patients affected by a rare genetic disease often face a diagnostic odyssey of misdiagnosis
and multiple visits across specialties. Next generation sequencing technology coupled with a
multidisciplinary approach has now shortened the diagnostic journey for such patients.
INTRODUCTION
There are more than 7,000 rare diseases in the world,
80% of which are genetic in origin. Many patients
embark on a diagnostic odyssey, involving numerous misdiagnoses and visits to multiple medical specialists;
it takes an average of 5 years to end the odyssey and
achieve a diagnosis of a rare disease. 50% of patients with rare diseases are children, of whom 30% do not
live beyond their fifth birthday.
Benefits of a genetic diagnosis
Rare diseases are not always immediately identifiable
to all medical specialists. A clinical diagnosis of a
rare genetic condition may be established based on
the presence of physical features and/or biochemical
findings.
Further benefits of a genetic diagnosis include enabling
patients and their families to receive advice about
the familial and reproductive risks. Genetic testing is
a powerful tool in establishing a genetic diagnosis
for patients. However, not all patients can afford the
high costs of genetic testing. With developments in technology, the costs of genetic testing have been rapidly declining.
NEXT GENERATION SEQUENCING
From the ability to sequence a single gene at a time,
technology has advanced to be able to sequence
multiple genes in parallel; this is also known as next generation sequencing (NGS). Instead of eliminating
one gene at a time, NGS allows multiple genes,
with overlapping features, to be analysed at a time.
It is particularly helpful in conditions with great
heterogeneity and a wide phenotypic spectrum.
NGS is available in the form of targeted panels,
exome sequencing, or genome sequencing. Exome
sequencing is a method of identifying possible disease-causing
variants by sequencing protein-coding exons
in the genome, while genome sequencing includes
areas outside of the protein-coding regions. However,
a key limitation is the analysis of the identified variants;
the greater the area sequenced, the more variants
identified for analysis, interpretation, and correlation
with the patient’s presentation.
Targeted panels using NGS technology have been
especially beneficial for genetically heterogeneous
conditions with multiple overlapping phenotypes.
In Singapore, rare diseases are defined as conditions
that affect fewer than one in 2,000 people. 6–8%
of the population are affected by a rare disease,
many of whom have faced, or are currently on, a
diagnostic odyssey. In addition, serial genetic testing
in a stepwise manner may incur higher costs with no
definitive diagnosis for the patient and their families.
As the cost of testing decreases, more patients can
access genetic testing and shorten their diagnostic
odyssey.
A genetic diagnosis can impact medical management,
such as directing the patient to relevant surveillance
of other organ systems, if necessary, prior to the development of symptoms.
In attempts to shorten the diagnostic journey for patients with rare diseases, new initiatives utilising NGS technology together with a multidisciplinary approach have been established.
1. BRIDGES
Improving access to genetic testing
The research programme BRIDGES (Bringing
Research Innovations for the Diagnosis of
GEnetic diseases in Singapore) was established
in 2014 with the primary aim of utilising genomic
technologies in collaboration with genomic
research institutes (Duke-NUS and A*STAR)
to benefit patients and improve their health
outcomes.
Over 400 patients and their family members
were recruited to have their genome sequenced.
It involved the collection of clinical information
and family history, review and prioritisation of
identified variants as well as clinical correlation.
Functional validation of the findings was
conducted where applicable, to support the pathogenicity of the variants in some families.
For patients who had either exome or genome
sequencing, there was an overall diagnostic
yield of 38.4%. In addition, cases with trio
(proband and biological parents) or quad
(proband, biological parents and biological
sibling) sequencing yielded a better diagnostic
rate than proband only or duo sequencing
(proband and one first-degree relative).
Over the years, BRIDGES has allowed patients
with previously undiagnosed diseases to access
genetic testing and receive a genetic diagnosis,
some of which have impacted their medical
management. With genetic results, families can
also be counselled about the recurrence risk and some have gone on to have unaffected children.
2. RAPIDSEQ
Targeting critically ill patients in NICU and
CICU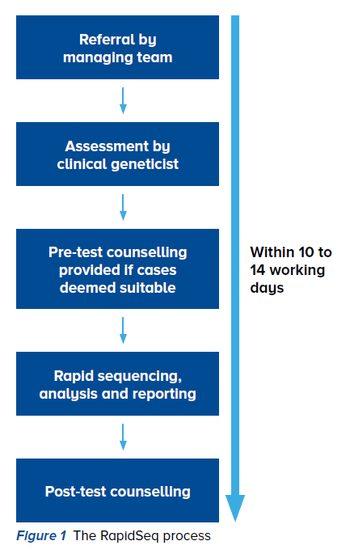
A genetic diagnosis is particularly critical for
the 35% of patients who will not survive their
first year of life due to a rare disease. With the
aim of bringing research capabilities to clinical
services, a translational program, Rapid Next
Generation Sequencing (RapidSeq), was
launched in April 2018 at the KK Women’s and Children’s Hospital (KKH).
It is targeted at critically ill patients in the
neonatal or paediatric intensive care units (NICU
or CICU) with suspected underlying genetic
conditions, with or without multiple congenital
anomalies (defined by the involvement of
two or more major organ systems or foetal
malformations detected on foetal imaging)
related to the suspected diagnosis.
RapidSeq is a multidisciplinary effort that
involves sequencing and analysing targeted
exonic regions of approximately 4,800 genes
associated with human diseases, also known
as a clinical exome. It hinges on the expertise
of the clinical team, including physicians,
genetic counsellors, laboratory staff and bioinformaticians, to meet the turnaround time
of 10 to 14 working days (Figure 1).
The clinical team first needs to assess and
phenotype the patient, establishing a list of
differential diagnoses, before obtaining consent
for RapidSeq. The samples are processed and
sequenced by the laboratory, and a list of
variants generated will be filtered and prioritised
using in-house computational algorithms by the bioinformatics team.
If a result is obtained, it will be disclosed to
the primary care team and parents, and a
preliminary report will be issued. Confirmation
via Sanger sequencing will be done before a
final report is issued. If no preliminary results
are obtained, other tests as per the clinical
indication may be considered, including exome
sequencing.
In the pilot phase, the first 10 cases saw a
high diagnostic yield of 40% within a short
turnaround time.
The provision of a genetic diagnosis in critically
ill patients can be beneficial through the
modification of existing treatments, as well as
the reduction of unnecessary investigations,
some of which may be invasive.
Should the diagnosis be one of poor prognosis,
it can also help the clinical team support the
family through decisions such as treatment
limitation and palliative care. Apart from that,
the family can also benefit from information
about the recurrence risk.
SINGHEALTH DUKE-NUS GENOMIC MEDICINE CENTREBringing genomic expertise across SingHealth for more accessible careEstablishing a genetic diagnosis may end the
diagnostic odyssey, but that is merely the tip of the
iceberg. Patients with rare genetic syndromes often
require care from multiple specialties. At present,
care tends to be fragmented and not standardised due to differing practices and management between
healthcare institutions.
The SingHealth Duke-NUS Genomic Medicine Centre
(SDGMC) brings together genomic expertise across
SingHealth institutions to enable more accessible
care for patients with rare genetic diseases. Standardised care across SingHealth institutions
The SDGMC endeavours to enhance the patient
experience and provide standardised care for patients with genetic conditions across SingHealth. The establishment of specialty genetics clinics would enable patients to receive appropriate genetics support at the institution where they are receiving care. Coordinating care across specialties
There would also be more coordinated care and support plans, for patients and families, as communication between various specialities are improved. In addition, opportunities for closer collaborations are created, and research findings are accelerated by breaking down disease boundaries and investigating clinical challenges in a holistic and efficient manner. |
RARE DISEASES ARE ACTUALLY NOT THAT RARE
As more rare diseases continue to be discovered each year, they are collectively not that rare. Leveraging on current technology and genomic advances will allow
us to provide better medical care support for patients and families with rare genetic diseases.
Primary care doctors play an important role in
screening during a patient’s regular visit. Patients
with suspected undiagnosed genetic conditions, or
individuals with family history of specific genetic
conditions, should be referred to a specialty genetics
clinic or genetics service for evaluation and genetic
counselling.
REFERENCES
- Boycott KM et al. International Cooperation to Enable the Diagnosis of All Rare Genetic Diseases. Am J Hum Genet. 2017 May 4; 100(5): 695–705.
- Spotlight on rare diseases. Lancet Diabetes Endocrinol. 2019 Feb; 7(2):75.
- Posey JE. Genome Sequencing and Implications for Rare Disorders. Orphanet J Rare Dis. 2019 Jun 24; 14(1):153.
- Shashi V et al. The Utility of the Traditional Medical Genetics Diagnostic Evaluation in the Context of Next-Generation Sequencing for Undiagnosed Genetic Disorders. Genet Med. 2014 Feb; 16(2):176–182.
- Daoud H. Next-generation sequencing for diagnosis of rare diseases in the neonatal intensive care unit. CMAJ. 2016 Aug 9; 188(11):E254–E260.
- Ting TW and Jamuar S. On the Fast Track – Rapid Genomic Sequencing for Critically-ill Children. Special Delivery Newsletter. 2019 Apr 1; https://www.kkh.com.sg/news/patient-care/on-the-fast-track-rapid-genomic-sequencing-for-critically-ill-children#
- Ministry of Health Singapore. Rare Disease Fund to Provide Financial Support to Singaporeans With Rare Diseases. 2018 Nov 19; https://www.moh.gov.sg/news-highlights/details/rare-disease-fund-to-provide-financial-support-to-singaporeans-with-rare-diseases
Sylvia Kam is a Genetic Counsellor at the SingHealth-Duke NUS Genomic Medicine Centre. She is part of the teams at the KK Women's and Children's Hospital Genetics Service as well as the SingHealth Duke-NUS Institute of Precision Medicine. She received her Master of Genetic Counselling from the University of Melbourne, Australia, and is a Member of the Human Genetics Society of Australasia (MHGSA). Apart from her clinical interests in paediatric and inherited conditions, she also has an interest in the curation of genetic variants. She is currently a member of the ClinGen Syndromic Disorders Working Group as a biocurator.
GPs who would like more information on this topic, please contact Ms Kam at [email protected].
Dr Koh Ai Ling is an Associate Consultant Paediatrician in the Genetics Service, Department of Paediatrics at KK Women’s and Children’s Hospital. Her clinical and research areas include rare genetic disorders and inherited metabolic disorders. She has a strong interest in medical education and provides regular genetics and metabolic teaching for paediatric residents. She currently serves as a trainer in the Genetics Education for Healthcare Professionals workshop at SingHealth Academy which focuses on the application of a wide range of genetic tests, and the understanding of roles in genetic counselling and the legal framework regulating genetic testing.
GPs who would like more information on this topic, please contact Dr Koh at [email protected].
Lim Jiin Ying received a Bachelor of Science (double degrees in Genetics and Biochemistry and Molecular Biology), and a Master's in Genetic Counselling from the University of Melbourne. She joined the Genetics Service at KK Women’s and Children’s Hospital as a Genetic Counsellor in 2013. She works closely with families to provide genetic counselling and support. Her clinical interests include prenatal, paediatric, metabolic and genodermatoses. In addition to her clinical role, she has also taken an active interest in research including the genetic basis of rare disorders and genetic counselling processes in next generation sequencing technology.
GPs who would like more information on this topic, please contact Ms Lim at [email protected].
GPs can call the SingHealth Duke-NUS Genomic Medicine Centre for appointments at the following hotlines:
KK Women's and Children's Hospital: 6692 2984
National Cancer Centre Singapore: 6436 8288
