Fertility preservation is often not foremost in the minds of women who have been diagnosed with cancer, or even after treatment. However, as cancer survival rates for children and young adults improve, the demand for fertility preservation services will increase. General practitioners who see such patients can play an important role in sharing with them the options available.
INTRODUCTION
Women have a finite reproductive lifespan. A baby girl
is born with all the follicle-containing oocytes she will
have in her lifetime – a finite supply which is depleted
with time as the follicles undergo atresia, and is
completely exhausted at menopause.
In addition, there is also a known decline in oocyte
quality in terms of aneuploidy, related to age-related
instability of the oocyte meiotic spindle. This results
in ovarian ageing and its clinical consequences of
infertility, miscarriage and pregnancy with a Down
syndrome foetus.
WHEN SHOULD MEDICAL FERTILITY
PRESERVATION BE CONSIDERED?
Chemotherapy, pelvic irradiation and ovarian surgery
are iatrogenic factors that are known to accelerate the
natural decline in ovarian reserve. The devastation to
the ovarian reserve and resulting risk of premature
menopause depends on the type of treatment and
its ovarian toxicity, the age of the woman and her
baseline ovarian reserve.
Hence, timely referral to a gynaecologist who specialises in reproductive medicine is crucial when
a reproductive-aged woman requires chemotherapy
or pelvic irradiation, or has undergone ovarian
surgery.
Patient criteria
Women who are suitable candidates for medical
fertility preservation have the following characteristics:
-
Age ≤ 40 years
- Premenopausal
- Has a realistic chance of surviving for five years
- Not pregnant
- Desires to have a child in the future
What Are the Options for Female Fertility Preservation?
1. EGG / EMBRYO FREEZING
Ovarian stimulation followed by the freezing of
mature eggs or embryos is the most established
method of fertility preservation.
Process
The patient self-administers subcutaneous gonadotropins
for about 10-14 days to stimulate multifollicular
development in her ovaries, following
which she undergoes an egg retrieval procedure
under sedation. The patient would be fit to tart chemotherapy as early as two days after
egg collection.
If there is no male partner, the eggs are supercooled
and stored in tanks containing liquid nitrogen.
If the woman is married, the eggs can be fertilised
and the resultant embryo cryopreserved. The
process is essentially similar to in-vitro fertilisation
(IVF), the main difference being that the embryos
are frozen rather than transferred in utero.
Potential risks and complications
The risk of complications is low and includes the
following:
-
Ovarian hyperstimulation syndrome
- Venous thromboembolism
- Procedural risks associated with egg collection – bleeding, infection, ovarian torsion
Oestrogen-sensitive tumours and breast cancer
recurrence
In patients with oestrogen-sensitive tumours such
as breast cancer, aromatase inhibitors are given
concurrently to alleviate the supraphysiological
estradiol levels during ovarian stimulation. Women
who were given letrozole during ovarian stimulation
had the same risk of breast cancer recurrence as
for those who did not undergo ovarian stimulation, wthout compromise in stimulation results.1
Ostetric and perinatal risks
No increased obstetric and perinatal risks were
found in pregnancies achieved with frozen eggs
or embryos, compared with IVF pregnancies
conceived with fresh eggs or embryos. However,
the long-term health of babies born as a result of gg freezing is not known.
Although the risk of miscarriage and chromosomal
abnormality is related to the age at which the eggs
were retrieved and frozen, egg/embryo freezing
does not prevent the other obstetric complications
(e.g., eclampsia, gestational diabetes, growth
restriction, caesarean section) associated with
advanced maternal age.
For example, a 45-year-old woman attempting to
conceive with eggs that were frozen when she was
at age 30 would have the same risk of maternal
and perinatal complications as women in her
current age group. However, her risk of miscarriage
and chromosomal abnormality would be similar to
that of a 30-year-old.
Efficacy
The success of female fertility preservation is
highly dependent on the woman’s egg quality
and ovarian reserve, which are in turn significantly
influenced by age.
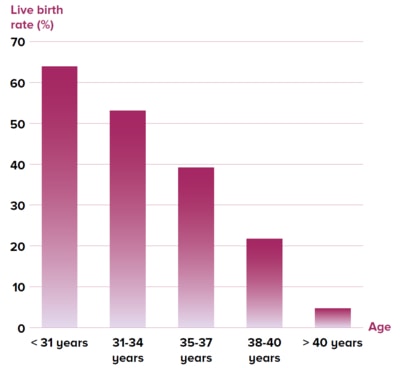
Figure 1 Live birth rate stratified by age after
one complete cycle of embryo freezing in 20,687
Chinese women2
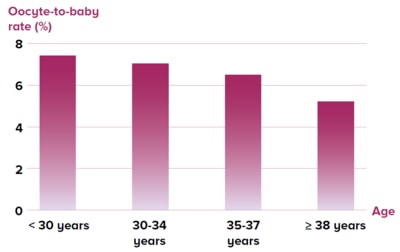
Figure 2 Oocyte-to-baby rate stratified by age3
A large study2 reported the live birth rate after
one cycle of embryo freezing in 20,687 Chinese
infertile women of various ages (Figure 1),while
another study3 reported the oocyte-to-baby rate
for different ages of women (Figure 2).
Besides egg quality, other factors such as smoking,
history of polycystic ovarian reserve and previous
ovarian surgery are also important.
A common misconception is that fertility preservation
is an insurance against future infertility; it is
not. The reality is that live birth is not a guarantee,
and it is more realistic to consider fertility
preservation as offering an extra opportunity to conceive with younger and better-quality gametes.
2. OVARIAN TISSUE FREEZING
Ovarian tissue freezing offers a different approach
to fertility preservation, other than the freezing of
mature eggs or embryos.
A typical cycle of egg freezing allows the retrieval
of a small number of eggs (usually less than
30), whereas the freezing of ovarian tissue with
whole follicles, each containing a single oocyte
surrounded by steroid hormone-producing cells, allows thousands of oocytes to be frozen in one
instance.
Premature ovarian failure is a known adverse
effect of highly gonadotoxic chemotherapy as well as pelvic radiotherapy. As ovarian tissue freezing
also preserves the steroid hormone-producing
cells of the follicular unit, it can restore fertility as
well as the hormonal function of the ovary.
Process
The ovarian tissue is retrieved by surgery, which
can usually be performed laparoscopically. As
most of the follicles in the ovarian tissue are in
the primordial stage, the ovarian tissue needs to
be reimplanted back into the body via a second operation to regain its functionality.
Typical graft sites are the remaining ovary and
pelvic sidewall (orthotopic) and anterior abdominal
wall (heterotopic). Currently, the only way to use
frozen-thawed ovarian tissue is in vivo; in-vitro
methods to retrieve mature oocytes from primordial
follicles are still being researched on.
Unlike in conventional organ transplants, patients
do not need to take any long-term immunosuppressive
medications after ovarian tissue
transplant surgery. This is because the ovarian
tissue that is harvested and reimplanted back into
the body is the patient’s own, thus there is no risk
of organ rejection.
Timing considerations
The lifespan of the graft is very variable, and
depends on the amount of tissue transplanted
and the age of the woman when the ovarian tissue was first removed. Graft survival ranging from a
few months to up to ten years has been reported.4
Given the limited lifespan of ovarian tissue grafts,
transplantation should be postponed until the
patient is ready to conceive or experiences
symptoms of ovarian hormone deficiency.
Efficacy
Ovarian function was restored in more than
95% of cases, within four to nine months after
transplantation. Among women who were trying to
conceive after ovarian tissue transplant, a live birth
rate of about 30% has been reported, of which half
were spontaneous conceptions.5
Ovarian tissue freezing is a relatively new
procedure, and its experimental label was removed
by the American Society of Reproductive Medicine
only as recently as 2019.
Overall, data on the efficacy, safety and
reproductive outcomes after ovarian tissue
freezing is still limited. It is currently considered
an ‘established medical procedure with limited
effectiveness’ that should be offered to carefully
selected patients.6
Indications
Ovarian tissue freezing is currently the only option
for prepubertal girls.
It can also be offered to patients undergoing
moderate- or high-risk gonadotoxic treatment (e.g.,
stem cell transplant, pelvic radiotherapy) or where
egg/embryo freezing is not feasible. For example,
patients who need to start cytotoxic treatment
urgently would not have time to undergo the
ovarian stimulation required for egg/embryo
freezing.
Potential risks and complications
In addition to the surgical complications of freezing
and grafting the ovarian tissue, there are concerns
regarding the presence of occult metastases in
the frozen ovarian tissue and retransplanting the
original malignancy. The risk is theoretical with
careful patient selection, and depends on the type
and stage of cancer.7
| Requires hormonal stimulation – delay in cytotoxic treatment needed | Does not require hormonal stimulation – no delay
in cytotoxic treatment |
| Does not require surgery; procedure (usually
transvaginal) done under sedation to retrieve eggs | Requires surgery (partial or total oophorectomy) performed under general anaesthesia; a second
operation is needed to reimplant the ovarian tissue
when fertility is desired |
| Smaller numbers of oocytes/embryos frozen
(usually less than 30) | Allows the freezing of thousands of oocytes at one
time |
| Mature oocytes or fertilised oocytes (i.e., embryos)
are frozen | Immature (primary) oocytes are frozen |
| Requires assisted reproductive technology | Allows opportunity to conceive spontaneously |
| Does not preserve ovarian hormonal function | Allows restoration of fertility and ovarian hormonal function |
| Risks related to ovarian stimulation and egg
collection | Surgical risks |
| More established method of fertility preservation | Less established method of fertility preservation |
Figure 3 Key differences between egg / embryo freezing and ovarian tissue freezing
3. GONADOTROPIN-RELEASING HORMONE (GnRH)
AGONISTS
Although GnRH agonists are commonly used
during chemotherapy to reduce the chance of
premature ovarian insufficiency, the mechanisms
which underlie this effect are uncertain.
Efficacy
In addition, studies have shown conflicting
results regarding the risk reduction for premature
ovarian insufficiency, with better results seen in
breast cancer patients compared to those with
haematological malignancies.
Finally, a reduction in premature ovarian
insufficiency may not result in higher fertility
rates.8 Very few studies on the use of GnRH
agonists for reduction of chemotherapy-induced
gonadotoxicity included pregnancy rates as an
end-point, thus evidence for the fertility-preserving
potential of GnRH agonists is scarce.
As such, international guidelines are unanimous in
stating that because of the limited evidence, GnRH
agonists should not be considered an equivalent
or alternative option for fertility preservation and
should not be used in place of proven fertility preservation methods.8
Patient background
A 24-year-old lady with early-stage Hodgkin’s
lymphoma was referred for fertility preservation.
She was single and virgo intacta with a good
ovarian reserve. She had regular periods with no
known gynaecological issues. The patient was planned to start urgent
chemotherapy with doxorubicin, bleomycin,
vinblastine and dacarbazine (ABVD).
Assessment and counselling
The patient was offered ovarian tissue freezing
as there was insufficient time for the ovarian
stimulation needed for egg freezing.
She was reviewed by the anaesthetist prior to
surgery and was assessed to be low-risk for
general anaesthesia. In addition to the operative
risks, the patient was also counselled about the
risk of ovarian metastasis and reimplantation
of the original malignancy with ovarian tissue
grafting, although the risk is small with Hodgkin’s lymphoma. Fertility preservation and chemotherapy
She underwent laparoscopic unilateral oophorectomy
and made an uneventful recovery from
the surgery. Chemotherapy was started two days
post-surgery. GnRH agonist was started on the day
of surgery and continued throughout the duration
of chemotherapy to reduce the risk of premature
ovarian insufficiency. |
TAKE-HOME MESSAGES FOR GPs
As cancer survival rates for children and young
adults improve and patients are looking to enhance
their quality of life after cancer survival, the demand
for fertility preservation services will increase.
General practitioners may occasionally see young
women who have been cured from cancer in their
practice.
It is important to be aware that these patients are
likely to have a reduced fertility potential compared
to their counterparts in the same age group. Many
of these patients may not have had the opportunity
to receive fertility counselling before starting cancer
treatment. Some of these patients may have been
offered fertility preservation at the point of diagnosis, but may have been too overwhelmed to pursue any
definitive fertility preservation procedures.
For cancer patients who are keen to start a family,
the fertility discussion does not stop with cancer
treatment. It is equally important to continue this
conversation after treatment has been completed,
given the limited reproductive lifespan of many of
these women.
As long as premature ovarian insufficiency has not
occurred, there is still a role for fertility preservation
after cancer treatment, although pregnancy
outcomes would be expected to be inferior to that
before starting cancer treatment.
REFERENCES
Rodgers et al. The safety and efficacy of controlled ovarian hyperstimulation for fertility preservation in women with early breast cancer: a systematic review. Hum Reprod. 2017 May 1;32(5):1033-1045.
Zhu et al. Live birth rates in the first complete IVF cycle among 20 687 women using a freeze-all strategy. Hum Reprod, 2018 May 1;33(5):924-929.
Doyle et al. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril. 2016 Feb;105(2):459-66.e2.
Jensen et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum Reprod. 2015 Dec;30(12):2838-45
Gellert et al. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet. 2018 Apr;35(4):561-570.
fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019 Dec;112(6):1022-1033.
Dolmans MM, Masciangelo R. Risk of transplanting malignant cells in cryopreserved ovarian tissue. Minerva Ginecol. 2018 Aug;70(4):436-443.
Blumenfeld Z. Fertility Preservation Using GnRH Agonists: Rationale, Possible Mechanisms, and Explanation of Controversy. Clin Med Insights Reprod Health. 2019 Aug 21;13:1179558119870163
Dr Serene Lim is a Consultant at Singapore General Hospital. She received her specialist
accreditation in obstetrics and gynaecology in 2015. In 2018, she was awarded the Health
Manpower Development Plan Scholarship by the Singapore Ministry of Health to pursue a
one-year fellowship in reproductive medicine and fertility preservation with Professor Kate Stern at the Royal Women’s Hospital, Melbourne. Dr Lim sees both general obstetrics and
gynaecology patients and has clinical interests in reproductive medicine, infertility, fertility
preservation, in-vitro fertilisation, pregnancy and labour care.
To find out more about our transplant programmes, GPs can contact the
SingHealth Duke-NUS Transplant Centre:
Tel: 6312 2720
Email: sd.transplant.centre@singhealth.com.sg
